
Vulvovaginal Pain Disorders
The Treatment of Vulvovaginal Pain Disorders with Surface Electromyographic Assisted Pelvic Floor Muscle Rehabilitation
Howard I. Glazer, Ph.D.
Clinical Associate Professor of Psychology in Psychiatry.
Clinical Associate Professor of Psychology in Ob/Gyn, Cornell University
Medical College.
Stanley C. Marinoff, MD,
M.Ph Director,
Center for Vulvovaginal Disorders, Washington, D.C. and
Clinical Professor of Obstetrics and Gynecology, George Washington University
School of Medicine and Health Sciences, Washington, D.C.
Introduction
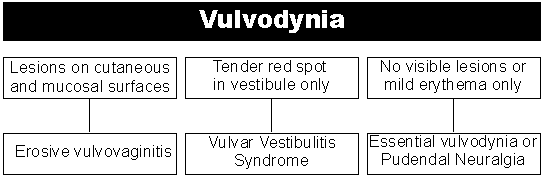
Vulvodynia is a descriptive, not a diagnostic, term covering a wide range of disorders which have, as one component, pain in the vulvar area. Vulvar pain can arise from many sources. In no other area of biofeedback practice, is it more important to rule out all medical reasons for the symptoms prior to commencing treatment, and to treat patients only under prescription from a specialty physician, not on self-referral. While there are many acute problems that may initiate or prolong introital dyspareunia (pain on vaginal penetration) or vulvar burning sensations, you will probably be referred only the chronic cases. If there are still acute problems present, these must be remedied prior to further treatment. Gynecological sources of vulvovaginal discomfort include vaginal infections, fungal (a wide range of yeast overgrowth includes hundreds of identified species) or bacterial (Bacterial Vaginiosis). These infections cause changes in the vaginal ecosystem such that vaginal discharge is highly irritative to the vulvar tissue. Another major source of vulvar discomfort is hormone related and occurs perimenopausally and post menopausally. Estrogen deficiency frequently leads to a thinning of vulvar tissue with consequent irritation. Dermatological sources of vulvar discomfort include a variety of conditions involving vulvar tissue changes such as lichen simplex chronicus, lichen planus and lichen sclerosis. In these conditions, a thickening of the vulvar skin, lichenification, causes localized irritative symptoms. Venerological sources of vulvovaginal pain encompass all sexually transmitted diseases such as herpes simplex virus (HSV). Vulvar warts, such as those found in human pappilomavirus (HPV) are known to cause irritation in the vulva. Precancerous and cancerous vulvar diseases, including vulvar intraepithelial neoplasias (VIN, precancerous) and warty and baseloid vulvar carcinomas, are all known to cause vulvar discomfort. Many women experience transient vulvar irritation from any of the above sources or from contact with irritants, including soaps, detergents, topical vulvar preparations used to treat some of the above conditions, prolonged or inadequately lubricated penile vaginal intercourse and vulvar trauma associated with accidents or surgery. In most cases, the irritation is transient and does not need to be addressed once the underlying causes have been identified and treated. This extremely limited overview of the sources of vulvar irritative symptoms is given to emphasize the necessity for a complete diagnostic work up and appropriate medical treatment before any biofeedback intervention is considered.
Once all known sources of vulvar tissue disturbance
have been identified, diagnosed and satisfactorily treated, there remains
a population of women in which vulvar irritative symptoms persist. The
remaining two symptom groupings are just that, not diagnoses but symptoms
and are, by definition, diagnosed by exclusion. They do not have a known
etiology, are not associated with any known tissue pathology and therefore
are "functional" in nature, as opposed to "structural".
These two, often overlapping, symptom patterns include:
- Vulvar Vestibulitis Syndrome
- Essential or Dysesthetic Vulvodynia.
Differential
Diagnosis
Vulvar vestibulitis syndrome (VVS) is characterized by introital dyspareunia and may involve swelling, erythema and exquisite tenderness to touch localized to the vestibule of the vagina (anatomically defined as extending from the frenulum of the clitoris, anterioly, to the forchette, posteriorly. The innermost border is the hymenal ring and the lateral border is Hart's line on the inner aspect of the labia minora. Into this space, open the major vestibular glands - Bartholin's, Skene's and periurethral - and the minor vestibular glands). The term vulvar vestibulitis syndrome was introduced in 1987 by Eduard Friedrich (1) to group together a constellation of signs and symptoms that involved, and were limited to, the vulvar vestibule. These consist of 1) severe pain on vestibular touch or attempted vaginal entry, 2) tenderness to pressure localized within the vulvar vestibule, and 3) physical findings confined to vulvar erythema of various degrees. Patients with this condition typically suffer no discomfort unless there is direct pressure on the vestibule. It is quite usual for these patients to avoid wearing tight jeans and situations involving prolonged sitting, which would exacerbate the pressure of tight clothing. These patients are often intercourse abstinent and, eventually, totally sexually abstinent for prolonged periods of time. Since the demographics of these patients are typically Caucasian, educated, upper-middle class women ranging in their 20's and 30's, the sexual consequences of this condition are often psychologically and interpersonally devastating. Because this condition has no known etiology and is symptomatically manifest and functionally limiting primarily in the area of sexual activity, it is not surprising that some have suggested that this is a psychogenic disorder. Research in this area has demonstrated clearly that this population shows no significant medical, psychological or sexual history differences from normal matched controls thus ruling out this hypothesis (2). Conservative medical treatment for this condition includes low dose tricyclics, to block the nerve mediated pain, antihistamines, to reduce the localized swelling, alpha interferon injections, topical palliatives such as oilated oatmeal (aveeno solution) and topical anesthetics (lidocaine), to permit intercourse. If these interventions produce unsatisfactory results, the Gold Standard treatment has been the surgical excision of the affected area, a skinning vestibulectomy with vaginal advancement and perineoplasty. The literature indicates that this procedure produces satisfactory results in approximately 80% of those treated (3).
Essential or dysesthetic vulvodynia is a condition of diffuse vulvar burning, which can vary from mild to extreme, and from intermittent to chronic. It tends to be progressive with respect to chronicity and intensity of symptoms. It is of unknown etiology and may have no visible vulvar changes. In its more intense and chronic form, it can be totally disabling. The term vulvodynia was first introduced at the 1975 Congress of the International Society for the Study of Vulvovaginal Diseases (ISSVD). In 1983 the ISSVD task force charged to study vulvar pain defined vulvodynia as "chronic vulvar discomfort, especially that characterized by the patient's complaint of burning (and sometimes stinging, irritation or rawness)". Like vestibulitis, it tends to reduce sexual activity, leading frequently to sexual abstinence and the associated psychological and interpersonal consequences. Medical treatments for this condition include tricyclics, antihistamines, neurontin, flexaril and topical palliatives and anesthetics. Surgery has not been shown to have any beneficial role in the treatment of this condition. While the subjective complaint in both VVS and vulvodynia may be dyspareunia, the sine-qua-non of pure VVS is introital dyspareunia. Patients with vulvodynia will also have constant or intermittent burning.
Vulvar vestibulitis syndrome and dysesthetic vulvodynia frequently overlap and are also frequently associated with interstitial cystitis (IC), a urological condition of urinary urgency, frequency and bladder spasms, irritable bowel syndrome (IBS), and fibromyalgia, a condition encompassing pervasive muscle pain and sleep disorder. This has led some to suggest a possible autoimmune source for these conditions. It has also been suggested that vestibulitis and vulvodynia are, possibly, different stages of the same disorder, with localized symptoms of vestibulitis becoming more persistent and diffuse as the irritation is prolonged. There are many problems with this hypothesis, such as patients who have prolonged vestibulitis without ever developing vulvar dysesthesia, and those, whose condition starts with dysesthesia but never develop localized introital dyspareunia. Another suggestion is that both of these conditions represent a form of vulvar reflex symathetic dystrophy with sensitization of nociceptive 'c' fibers, so that touch sensation is replaced with an experience of pain.
Unfortunately, due to limited funding and the relatively small percentage of the population affected by these conditions, little basic science or clinical research has been, or is presently, being conducted concerning these conditions.
Biofeedback
Methodology
The specialty physicians of the Columbia University College of Physicians and Surgeons "Cutaneous Vulvar Clinic" first approached Dr Glazer in 1991. They had noticed that during intravaginal digital palpation of the pelvic floor muscles of these women, there was considerable chronic tension and spasticity. These specialists requested the use of biofeedback to correct this muscle abnormality. Dr Glazer's initial reaction was to inform them that he did not believe that biofeedback would be useful in treating these conditions. He felt that the muscle hypertonicity most likely represented a "guarding" or "splinting" of the local striate muscle in response to the vulvar pain, and did not play a causative role in the pain. Due to the persistence of the referring physicians, Dr. Glazer began to work with this patient population using the standardized protocols and treatment regimes developed for the urological disorders of incontinence (4). It was immediately noticeable that the surface electromyographic (sEMG) patterns of this population's pelvic muscles showed abnormal tension and instability at rest, as well as weakness and instability during phasic, tonic and endurance voluntary contractions. After a period of "trial and error" in working with these patients, Dr. Glazer began to turn his focus away from the most prominent features of this sEMG pattern, the weakness displayed in the contractile amplitude. This came about because several patients had significantly increased their contractile amplitude but still showed no symptomatic benefit and, in fact, were sometimes worse. It appeared that the resting amplitude (excess tension) was most associated with vulvar pain. Focusing training on reducing resting amplitude proved to be more successful but still left a sizable portion of the population with little to no benefit. It began to emerge that the variability of the resting amplitude, and not just the amplitude, was a critical determinant of pain reduction. The stability of the signal became more significant than its amplitude and the resting signal more significant than the contractile signal. It appeared, empirically, that the variability of the resting signal and, secondarily, the variability of the contractile signal, were more closely associated with pain variations than signal amplitude characteristics. Of course, signal variability measures were clearly noted to vary directly in proportion to amplitude, i.e. higher signal amplitudes are more variable, both at rest and during contractions.
Research
The first publication using sEMG assisted rehabilitation of pelvic floor musculature in the treatment of vulvovaginal pain is entitled "Treatment of Vulvar Vestibulitis Syndrome with Electromyographic Biofeedback of Pelvic Floor Musculature" (Glazer et al 1995) (5). This study demonstrated a slightly more than 50% cure rate with an average self reported improvement of 83% with 80% of sexually abstinent patients resuming regular intercourse. Two main findings emerged statistically. The first being that there were neither demographic nor sEMG characteristics on initial evaluation which predicted response to this treatment modality. Second it demonstrated that only changes in the standard deviation of the resting sEMG signal predicted pain change. This finding confirmed Dr. Glazer's anecdotal experience that the treatment is essentially a MUSCLE STABILIZING program. This paper also concluded that "The response to this therapy suggests that whatever the initial insult or etiologic factor, vulvar vestibulitis syndrome may be a result of autonomically mediated pain. This mechanism, as a final common pathway for multiple etiologies, may explain the lack of consensus on a single antecedent, despite consistency in symptomatology of the syndrome." So, the critical factor to emerge, which differentiates this treatment from previous applications of pelvic floor biofeedback, is a shift of emphasis away from tonic contractile amplitude and towards reducing muscle variability overall, predominantly at rest.
Although on the average, vulvovaginal pain patients have less contractile capacity, It was found that there is a subset of patients who were at treatment onset, not only very tense and unstable at rest but, despite this, were also quite strong (above 25mv on initial contractile evaluation). This population posed a challenge as the tradition in pelvic floor rehabilitation was to teach the patient to exclusively localize their contractions in the pubococcygeus muscle, to the exclusion of surrounding accessory muscle. Using this training procedure, it was simply not possible to produce a contraction of adequate amplitude to reliably release the resting tension. Dr. Glazer found that permitting these patients to use the naturally occurring contractions of internal obturator, lower abdominals and adductor longus muscles, supported and enhanced the amplitude of the pelvic floor contraction adequately to "break" the resting tension level. Thus the "Glazer" protocols require the individualized "testing" of the patient with different positions and the use of different combinations of accessory muscles which enhance, rather than interfere with, the correct use of the pelvic floor muscles. This procedure is briefly outlined in a paper entitled "Functional Rehabilitation of Pelvic Floor Muscles: A Challenge to Tradition" (Glazer & MacConkey, 1996) (6).
In a 1997 paper (White, Jantos and Glazer) (7), a cohort of 32 vulvovaginal pain patients were compared to a matched control group of normals and found several sEMG characteristics which reliably differentiated the two groups. Cutoffs for these sEMG characteristics were developed and are summarized as follows:
This paper reports on a case history of a patient with classic vulvodynia who produced an sEMG protocol well within normal limits. Upon return to the gynecologist for further medical evaluation, the patient was diagnosed with cytolytic vaginosis, treated and was free of discomfort within a few days. The authors conclude, in this paper, that sEMG may be the first objective methodology for forming a differential diagnosis between functional vulvovaginal pain syndromes and other non-diagnosed medical sources of vulvovaginal pain such as infections.
Several publications are now in preparation including validating pelvic floor muscle assessment by digital palpation using sEMG as the standard (8). This study shows that sEMG is more reliable and more predictive of pelvic floor dysfunction than is digital evaluation of pelvic floor function. Another study in preparation compares sEMG biofeedback, or cognitive behavioral pain management, to vestibulectomy in the treatment of pure VVS (9). Early results suggest that biofeedback efficacy is close to that of surgery and improves over time. Research is now underway on the pattern of sEMG changes observed over treatment with focus on power density spectral frequency analysis, rise and recovery times and coefficients of variability for rest and contraction periods.
Protocol
The "Glazer" protocol for pelvic floor muscle evaluation uses a five-segment evaluation sequence as follows:
- One minute rest, pre baseline.
- Five rapid contractions (Flicks) with 10 seconds rest between each (phasic).
- Five 10 second contractions with 10 second rests between each (tonic).
- A single endurance contraction of 60 seconds (endurance).
- One minute rest, post baseline.
This protocol is a similar sequence to that used in assessing pelvic floor muscles for incontinence. The difference is not in the sequence of muscle actions but the measurements taken. As mentioned earlier, the major goal, in treating incontinence, is increasing contractile amplitude to enhance external urethral sphincter closure pressures. For pelvic pain, we have found that amplitude changes are only a small part of the picture. In the "Glazer" protocol, for each contraction and relaxation period, integrated sEMG amplitude and standard deviation is measured. In addition, coefficients of variability (standard deviation divided by amplitude) are taken as measures of muscle stability, rise and recovery times are taken at initiation and termination of each contraction and spectral frequencies (either FFT's or zero crossings) are taken for each contraction. Another difference between the "Glazer" protocol and previous incontinence protocols is that accessory muscles (often monitored with a second sEMG channel on lower abdominals) are not necessarily minimized. Each patient is assessed with the use of different combinations of accessory muscles. This is done in order to determine the best balance between keeping the patient's focus on the internal "lifting" sensation and, at the same time, maximizing the use of the muscle contraction to result in a reduction in amplitude and variability of the subsequent rest period. We look for an exercise position, contraction type, contraction duration, and number of repetitions which maximize the exercise. All patients are started on two 20 minute exercise sessions a day, each one consisting of 60 repetitions of 10 second contractions alternated with 10 second relaxation phases. All patients are required to use home training devices and intra-vaginal sensors in the conduct of their home exercises. Patients return for office evaluations every two weeks for their second and third visits and then, monthly, for subsequent visits. The frequency of office visits is determined by the observation of sEMG changes by the clinician, and compliance of the patient in the conduct of home exercises.
Over time, with continued training, we look for increased contractile amplitudes and spectral frequencies with decreased contractile coefficients of variability and rise and recovery times. In relaxation measures, we look for reduced amplitude and reduced coefficients of variability. Amplitude changes are not enough and we have seen, as mentioned earlier, many patients showing improved contractile amplitude with reduced resting amplitude and little therapeutic benefit. We believe that the spectral frequencies, rise and recovery times and coefficients of variability are related to the predominant fiber type being recruited and the coordination of use of that fiber type. The critical combination of higher amplitude contractions with higher spectral frequency, faster rise/fall times and reduced coefficients of variability suggest a predominantly fast twitch (type II) fibers. In the presence of this phenomenon (increased fast twitch coordination), the consequence is reduced amplitude and variability during rest and a reduction of the hypertonicity and instability associated with chronic uncoordinated discharge of fast twitch fibers as seen in the resting sEMG of untreated vulvovaginal pain patients.
Conclusion
Free form observations of sEMG with or without direct pelvic muscle palpation does not comprise an adequate evaluation. Replicable protocols, applied to the patient over time are necessary to assess progress. Similarly, amplitude and standard deviation measures alone are not adequate to assess changes, spectral frequencies, rise and recovery times, coefficients of variability (amplitude corrected standard deviations) must all be observed to assure that correct rehabilitation of the pelvic floor muscle is taking place. For those trained in the traditions of incontinence, it is also important to remember that you must explore positions, use of accessories, contraction duration, and number of repetitions to best achieve the desired sEMG changes. While some patients will do better with exclusive pelvic floor contractions, others cannot achieve desired outcomes without the use of accessory muscles.
References
- Friedrich, E.G., Jr., Vulvar Vestibulitis Syndrome. J. Reprod. Med. 32:110-114, 1987.
- Meana, M., Binik, Y., Khalifé, S., and Cohen, D., Biopsychological Profiles of Women with Dyspareunia. Obstetrics and Gynecology 90:4:583-590.
- Marinoff, S.C., and Turner, M.L.C., Vulvar Vestibulitis Syndrome. Dermatologic Clinics 10:435-444, 1992.
- Perry, J.D. Software Standards for Perineometry, Biotechnologies, Portland, Maine, 1984.
- Glazer, H.I., Rodke, G., Swencionis, C., Hertz, R. and Young, A.W., Treatment of Vulvar Vestibulitis Syndrome with Electromyographic Biofeedback of Pelvic Floor Musculature. J. Reprod Med. 40:283-290, 1995.
- Glazer, H.I., and MacConkey, D., Functional Rehabilitation of Pelvic Floor Muscles: A Challenge to Tradition. Urol. Nurs. 16:68-69, 1996.
- White, G. Jantos, M., and Glazer, H.I., Establishing the Diagnosis of Vulvar Vestibulitis. J. Reprod. Med. 42:157-160.
- Romanzi, L., Polaneczky, M. and Glazer, H.I. A Simple Technique for Assessment of Pelvic Muscle Function as a Part of Routine Pelvic Examination; Validation by Surface Electromyography. Manuscript in preparation.
- Bergeron, S., Binik, Y.M., Khalifé, S. Pagidas, K. and Glazer, H.I. A Randomized Treatment Outcome Study of Vulvar Vestibulitis Syndrome. Manuscript preparation.
Copyright, 1997 The Biofeedback Federation of Europe