
Phantom Limb Pain
The Use of EIectromyographic and Temperature Biofeedback for Treatment of Cramping and Burning Phantom Limb Pain
Richard A Sherman,
Ph.D
LTC, Medical Service, US Army Chief,
Clinical Biometrics Service
Dept. of Clinical Investigation
Fitzsimons Army Medical Center Aurora , CO.
Introduction
Virtually everybody who has had an amputation reports feeling sensations which appear to emanate from the amputated portion of the limb. Most of the time, these "phantom" sensations are painless and of sufficiently low intensity to be no more than a mild distraction(9). The sensations are usually similar to those which would be felt in an intact limb, including warmth, itching, sense of position, and mild squeezing. Awareness of details of the limb's shape and perceived ability to move it tend to fade with time. However, almost all amputees report continuing to feel at least some phantom sensations throughout the remainder of their lives. When phantom sensations become intense enough for the amputee to define them as painful, they are called "phantom pains". The neural mechanisms which permit perception of phantom limbs are well recognized(3,4). Sensations reaching the brain are identified for location on the skin by the homunculus, in the sensory cortex, which contains a representation of the entire body surface. Thus, a pinch of the left index finger tip stimulates a location on the homunculus representing the left index finger tip.
If the finger was amputated and a signal was started by pressure, etc., anywhere along the remaining nerve paths between the finger stump and homunculus, the resulting sensation would seem to emanate from the finger tip because the paths do not change much after amputation and the brain has no way to know that the finger tip is not present.
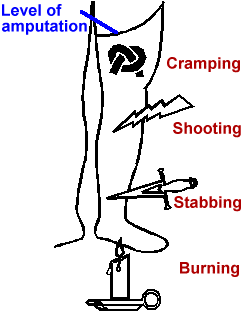
Figure 1. Common descriptions of phantom pain
Phantom limb pain occurs among between 50 and 80 percent of amputees(9,10). The most common descriptions of phantom pain are variants of cramping, burning/tingling, and shocking/shooting/stabbing (Fig. 1). Each amputee tends to report the same one or two descriptions of phantom pain whose locations within the phantom remain consistent over time. A large minority have episodes severe enough to interfere with work, sleep and desired social activities which occur frequently enough to require treatment.
Phantom pain can occur anytime, from just after an amputation to years later. Its occurrence is not related to psychological factors(12), age, sex, location of the amputation, or reason for the amputation (e.g. trauma vs. disease). Different individuals report their phantom pains to be affected by different environmental variables such as changes in humidity and temperature(2,10). As is true with all chronic pain syndromes, stress and fatigue can magnify the sensations but there is absolutely no evidence that any psychological factors cause phantom pain(1,12). Pain from pinched nerves in the back and other sources is referred to the phantom limb as it would be to the original limb.
Recent surface electromyographic studies(8,11) have demonstrated that the major muscles in the residual limb tense up several seconds before the cramping phantom limb pain begins and that these muscles remain tense for much of the duration of the episode. The pattern of tensing is usually shown by an abrupt increase in magnitude of surface electromyogram to about twenty times baseline values. Other studies(6) have demonstrated that burning phantom limb pain is closely associated with reduced blood flow in the residual limb. There is currently no evidence associating shooting/shocking descriptions of phantom pain with specific physiological mechanisms. However, very similar sensations are provoked during ectopic stimulation of nerves from a neuroma.
In the past, the success rate for treatment of phantom pain has been dismal, with only about one percent of treated amputees reporting effective relief lasting for at least a year(9,10). At least forty-three ineffective treatments were in common use until recently(10). They range in invasiveness from lobotomies and major spinal surgery, through surgical revision of the residual limb, psychotherapy, and psychoactive drugs, to transcutaneous electrical stimulation and similar techniques. The only treatments consistently able to ameliorate phantom pain were sympathetic locks and sympathectomies which were useful for burning phantom pain for up to a year.
Current treatments are based on the mechanisms discussed above and have been proven to be more effective(5,7). Cramping phantom pain responds well to treatments which result in preventing the residual limb from tensing up abnormally, while burning phantom pain responds well to treatments which increase blood flow, both in and out of the residual limb. No treatments have been identified as being consistently effective for shocking/shooting descriptions of phantom pain. The diagnostic decision making process for choosing the treatments most likely to be effective for different descriptions of phantom pain has been detailed elsewhere(3).
Biofeedback Treatment
For patients who describe burning/tingling phantom limb pain and have an essentially normal reactive vascular system, a trial of temperature biofeedback may provide relief. We start these patients out with surface electromyographic biofeedback because we find that it is easier to learn and gives trainees the confidence they need when learning to control their limb temperature. If the patient describes cramping/squeezing phantom limb pain, and is able to learn control of the voluntary muscles, a trial of surface electromyographic biofeedback is appropriate. Amputees who give mixed descriptions of phantom pain which include shocking/shooting sensations may have success learning to control other descriptions of phantom pain but the shocking/shooting sensations are likely to remain unchanged. Amputees reporting combined burning and cramping pain are given treatments aimed at controlling both underlying mechanisms.
The specific aim of the treatment is to teach amputees with burning/tingling phantom pain to habitually and unconsciously keep their residual limbs as warm as the intact limb. For amputees with cramping pain, the aim is to teach them to prevent the onset of the types of increases in muscle tension in the residual limb which lead to pain. These aims are approached through several overlapping stages:
- First, subjects are shown the relationship between the residual limb's temperature or muscular activity and the onset and intensity of phantom pain, so they are absolutely convinced of the relationship.
- Next, they are given muscle tension and temperature awareness training very similar to Jacobson's system. They are given tape recorded exercises to play at home at least twice per day. The aim of this phase is to begin increasing their awareness of changes in limb temperature and tension patterns as well as to begin helping them learn to control these parameters.
- After several weeks, the tapes are used only once per day and the patients begin doing the exercise on their own at least once at home and once while out in their normal work environment. This is intended to begin generalizing their awareness of changes in the parameters to their normal environment.
- The week after, subjects begin home training, they participate in weekly or biweekly biofeedback sessions conducted in the clinic. The sessions follow the guidelines detailed in the Association for Applied Psychophysiology and Biofeedback's application standards manual(14).
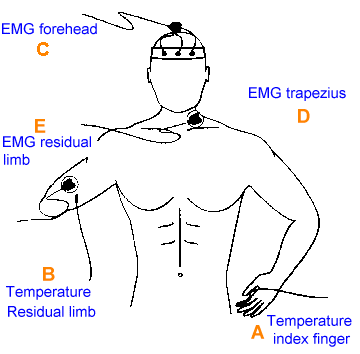
Figure 2. Placement of EMG and temperature sensors
 Figure 3. Bar graph |
|

Figure 5. Light bar feedback display
Conclusion
Biofeedback can be an effective treatment for cramping and burning phantom limb pain when used with appropriate patients in conjunction with home based temperature and muscle tension recognition and control training. This modality should be attempted before using medication or other invasive therapies as it is at least equally effective and relieves patients of the need for continuing use of drugs which usually have deleterious side effects.
REFERENCES
- Arena. J., R. Sherman, G. Bruno and J. Smith: The relationships between situational stress and phantom limb pain: Cross-lagged correlational data from six month pain logs. Psychosomatic Research, 34: 71-77 (1990).
- Arena, J., R. Sherman and G. Bruno: The relationship between humidity level, temperature and phantom limb pain: Preliminary Analysis. Biofeedback and Self-Regulation, 14: 128 (1989).
- Sherman, R.: Phantom limb and stump pain. In Neurologic Clinics of North America. (Portenoy. R. Ed.) Philadelphia, W.B. Saunders Co. (1989).
- Sherman, R., J. Arena and J. Ernst: The mystery of phantom pain: Growing evidence for physiological mechanisms. Biofeedback and Self-Regulation, 14(4): 267-280 (1990).
- Sherman, R., Arena, J., Griffin, V., Bruno, G., Cocilovo A.: Biofeedback for the treatment of phantom limb pain: An update. Biofeedback. 7(3): 7-8 (1991).
- Sherman, R. and G. Bruno: Concurrent variation of burning phantom limb and stump pain with near surface blood flow in the stump. Orthopedics, 10: 1395-1402 (1987).
- Sherman, R., N. Gall and J. Gormly: Treatment of phantom limb pain with muscular relaxation training to disrupt the pain/tension cycle. Pain 6: 47-55 (1979).
- Sherman, R., V. Griffin, C. Evans and A. Grana: Temporal relationships between changes in phantom limb pain intensity and changes in surface electromyogram of the residual limb. International Journal of Psychophysiology, In Press, 1992.
- Sherman, R. and C. Sherman: Prevalence and characteristics of chronic phantom limb pain among American veterans. Am. J. Phys. Med. 62: 227-238 (1983).
- Sherman, R. and C. Sherman: A comparison of phantom sensations among amputees whose amputations were of civilian and military origins. Pain 21: 91-97 (l985).
- Sherman, R. and C. Sherman: Physiological parameters that change when pain changes: Approaches to unraveling the "cause-or-reaction" quandary. Bulletin of the American Pain Society. 1(4): 11-15 (1991).
- Sherman, R., C. Sherman and G. Bruno: Psychological factors influencing chronic phantom limb pain: An analysis of the literature. Pain 28: 285-295 (1987).
- Sherman, R., C. Sherman and L. Parker: Chronic phantom and stump pain among American veterans: Results of a survey. Pain 18: 83-95 (1984).
- Biofeedback Applications Manual, AAPB, 1991.
Acknowledgments and Disclaimer
Figure 1 was originally finalized by Karen Wyatt, Medical Illustrator
at Fitzsimons Army Medical Center, from a draft provided by the author.
The manuscript was reviewed by Vernice Griffin and Cecile Evans of the
Psychophysiology Laboratory at Fitzsimons Army Medical Center. The work
reported here was entirety supported by the U.S, Army and the Department
of Veterans Affairs. However, the opinions and assertions contained
in this manuscript are the private views of the author and are not to
be construed as official or as reflecting the views of the United States
Departments of Veterans Affairs, Army or Defense.
Copyright, 1997 The Biofeedback Federation of Europe
